Development of a safe and
high quality “AOF medium”
There are various types of regenerative medicine; however, what is common to almost all regenerative medicine is the inclusion of a “process of culturing cells in an ‘artificial environment’.” By culturing cells in an artificial environment, that is, a condition where culture media is imbued, the cells essential for regenerative medicine can grow.
The development policy that we value most is being an “Animal Origin Free (AOF) system that does not contain any animal-derived components, including those from humans, in the culture medium.” There are at least 4 possible advantages of being an AOF.
-
POINT1
There is no risk of infection with
unknown viruses or pathogens of
human or animal origin. -
POINT2
The absence of animal-derived
components minimizes
the possibility of anaphylaxis. -
POINT3
There are very small lot-to-lot
differences, meaning cells of the
same quality and specifications
can be manufactured stably. -
POINT4
All of the components are evident,
which makes it relatively easy to
modify the properties of MSC by
changing the components in the
medium and to provide high
reproducibility.
In addition to the above, there are other advantages associated, such as easy confirmation of conformity with “Standard for
Biological Ingredients”, and reduced burden of documentation at the time of applications for clinical studies. We will continue to
develop new AOF medium while pursuing, first and foremost, the safety of patients to whom these cells are administered and
corresponding therapeutic effects.
Three research data sets showing the merits of AOF medium
1. Characteristics of AOF medium
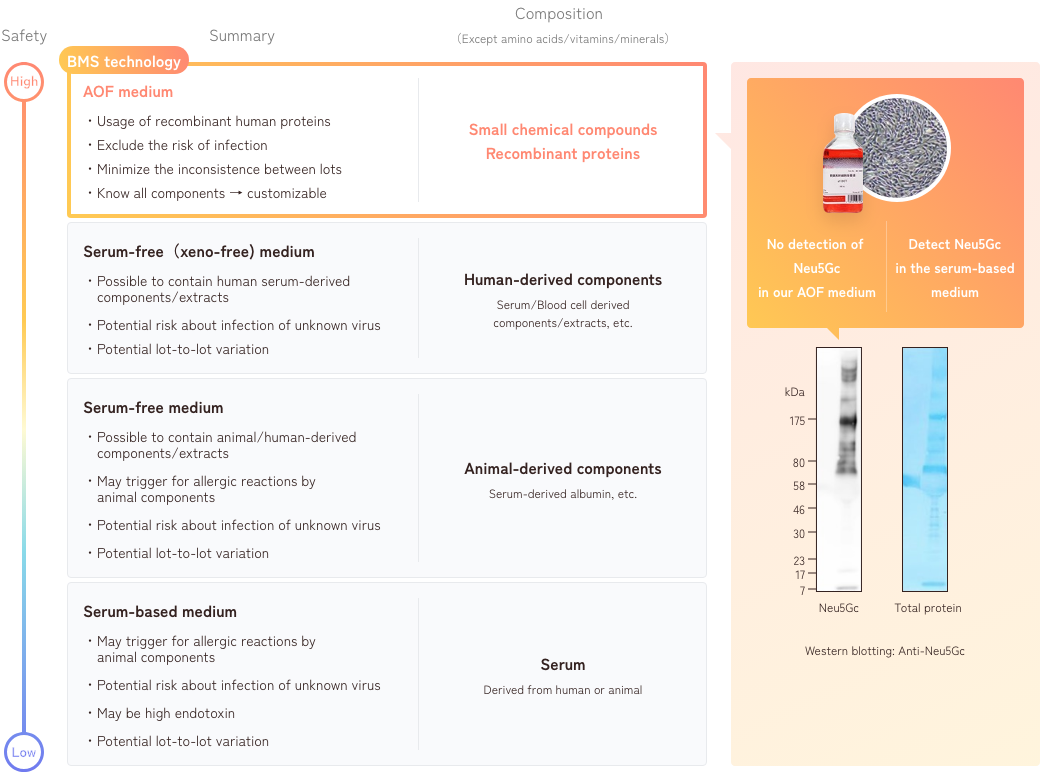
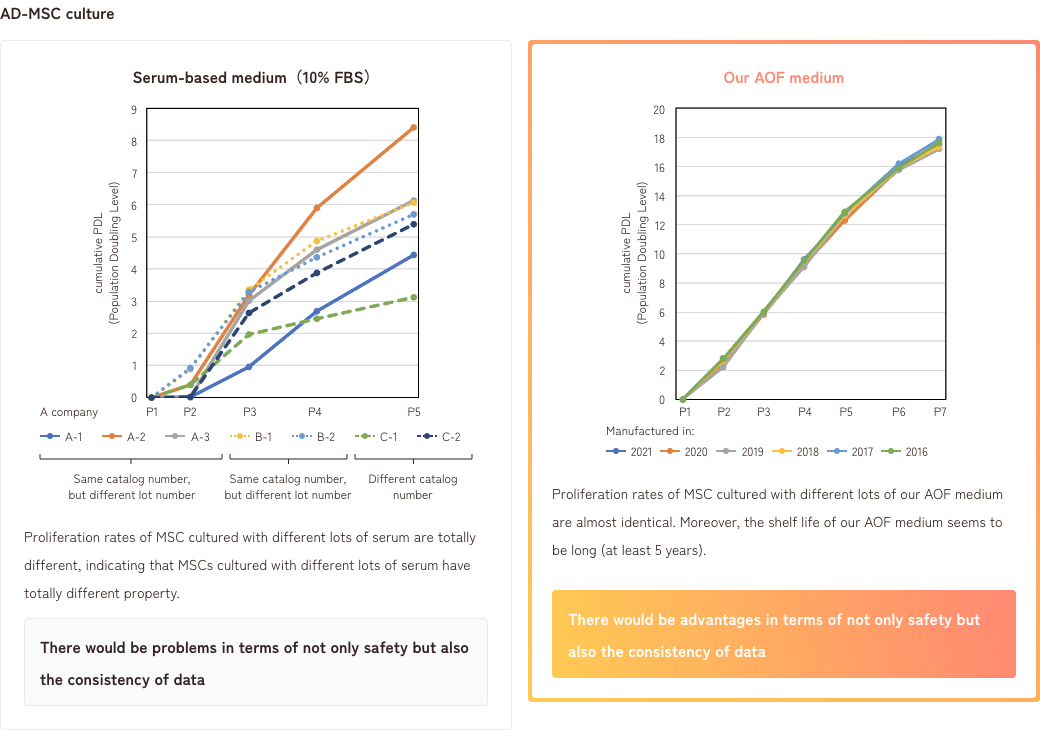
2. Differences between serum-based medium and AOF medium
3. Development of cells highly expressing specific functional factors
We have made a database of gene expression profile in MSC with several hundreds factors/chemicals, so that we can easily find the molecules changing the property of MSC
Five data sets indicating the safety and efficacy of our company’s medium
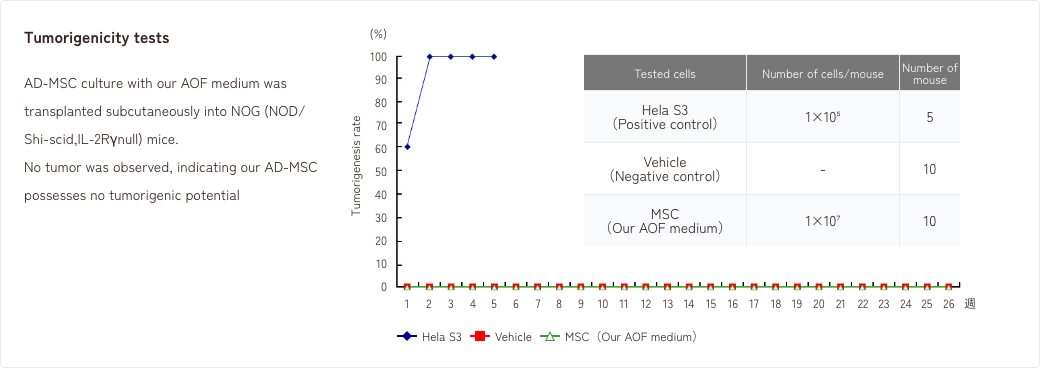
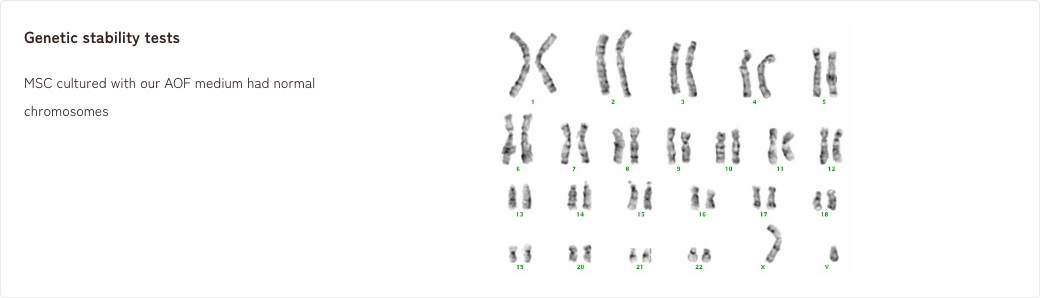
- 1. Tumorigenicity tests/Genetic stability tests
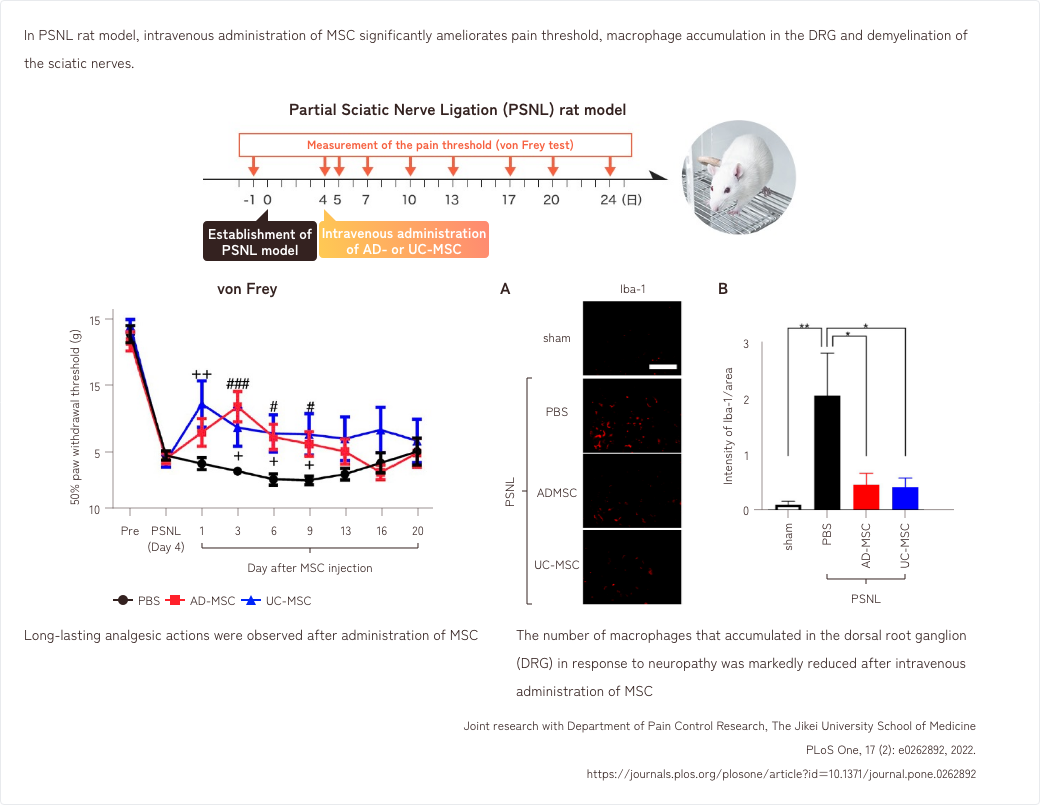
- 2. Effects of MSCs in a rat model of neuropathic pain
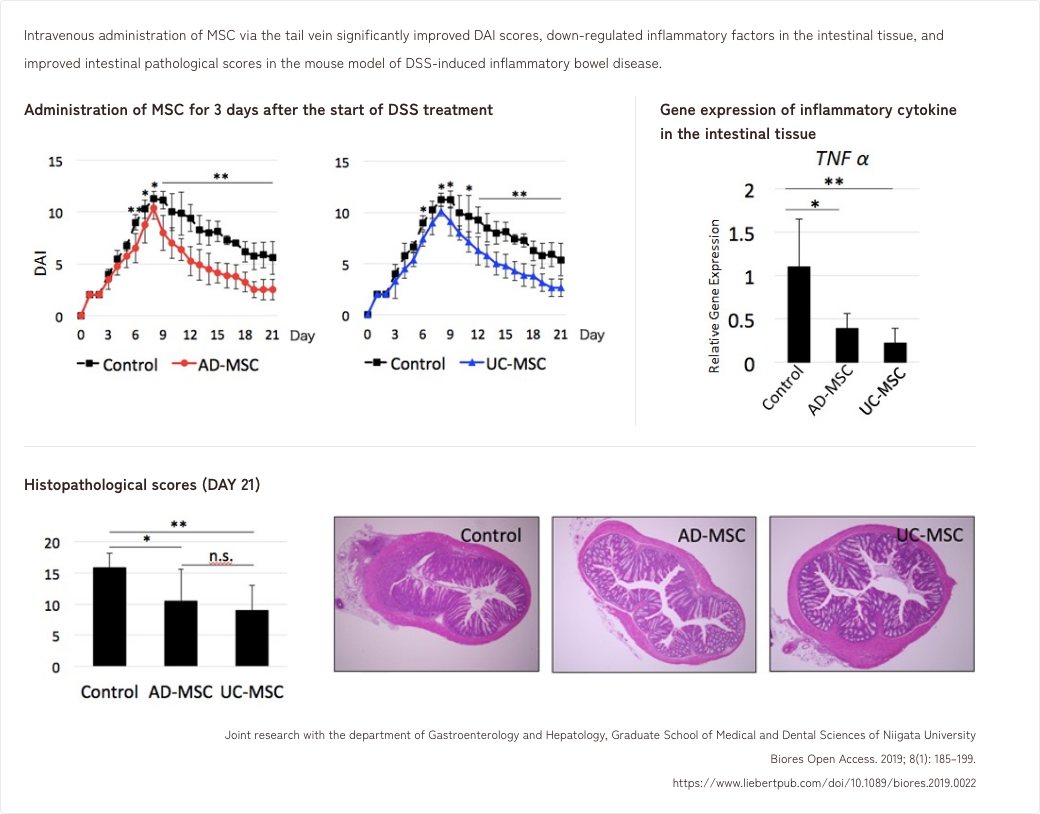
- 3. Effects of MSCs in a mouse model of DSS-induced inflammatory colitis
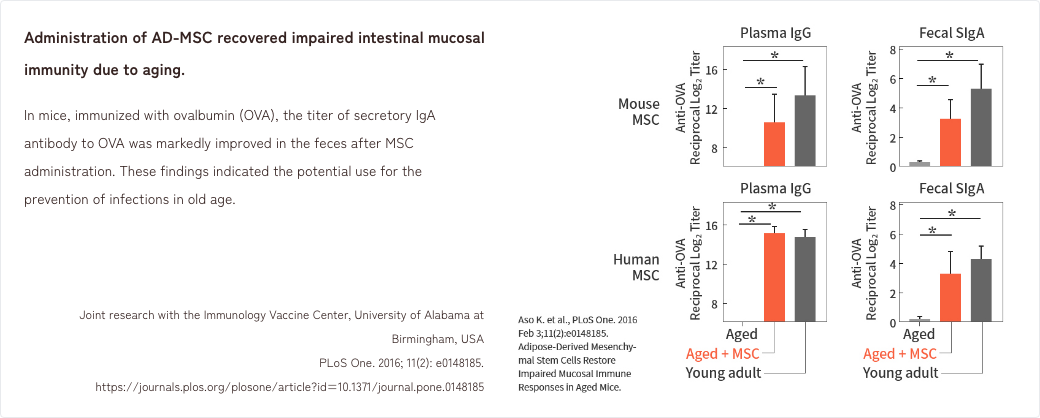
- 4. Effects of MSCs in an aged mouse model of intestinal mucosal immunity
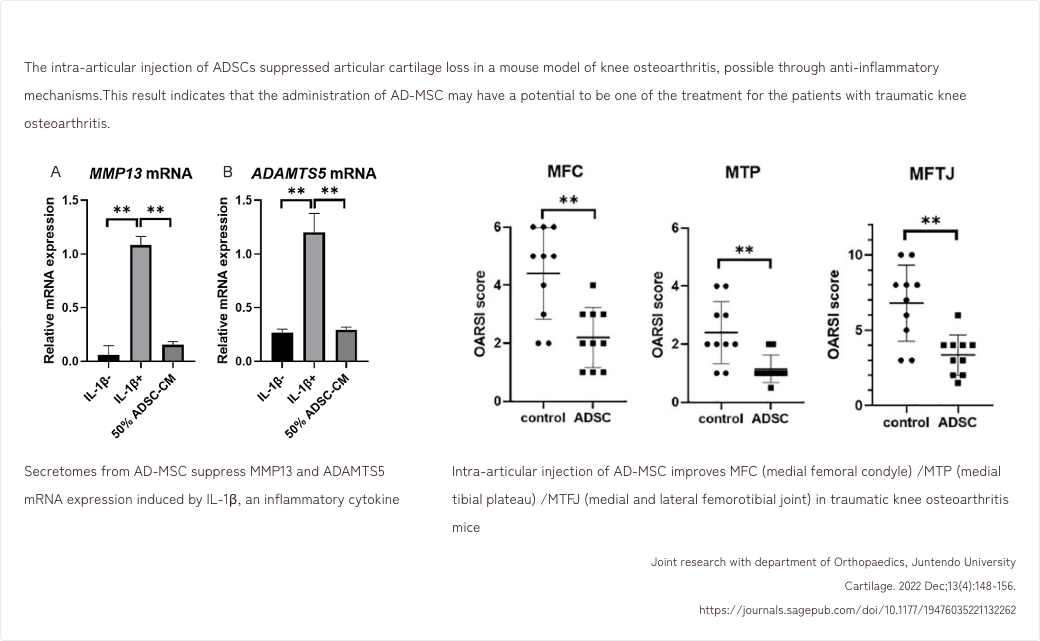
- 5. Effects of AD-MSCs in a mouse model of traumatic knee osteoarthritis
1. Tumorigenicity tests/Genetic stability tests
2. Effects of MSCs in a rat model of neuropathic pain
3. Effects of MSCs in a mouse model of DSS-induced inflammatory colitis
4. Effects of MSCs in an aged mouse model of intestinal mucosal immunity
5. Effects of AD-MSCs in a mouse model of traumatic knee osteoarthritis